
Yifan Su, Dexin Zheng, Lingfeng Ge, Le Yu,* David Lee Phillips, Jiani Ma,* and Yu Fang. Chem. Sci., 2024, DOI: 10.1039/D4SC06202G
Photoacid generators (PAGs) and photohydride generators (PHGs) are specific photolabile protecting groups that release acid and hydride, respectively. Over the past decade, great efforts have been devoted to developing novel PAGs and PHGs with advanced efficiency, among which, two of the promising candidates are the diarylethene (DAE)-based PAGs and PHGs, which release acids/hydrides during photochromic electrocyclization. The release quantum yields of PAGs can up to 50%, while PHGs are only 4.2% even after molecular structure modification. In this work, we reveal the photochemical reaction mechanisms of PAG (1o) and PHG (2o) using femtosecond/nanosecond time-resolved spectroscopy and DFT/TD-DFT calculations (Fig. 1).
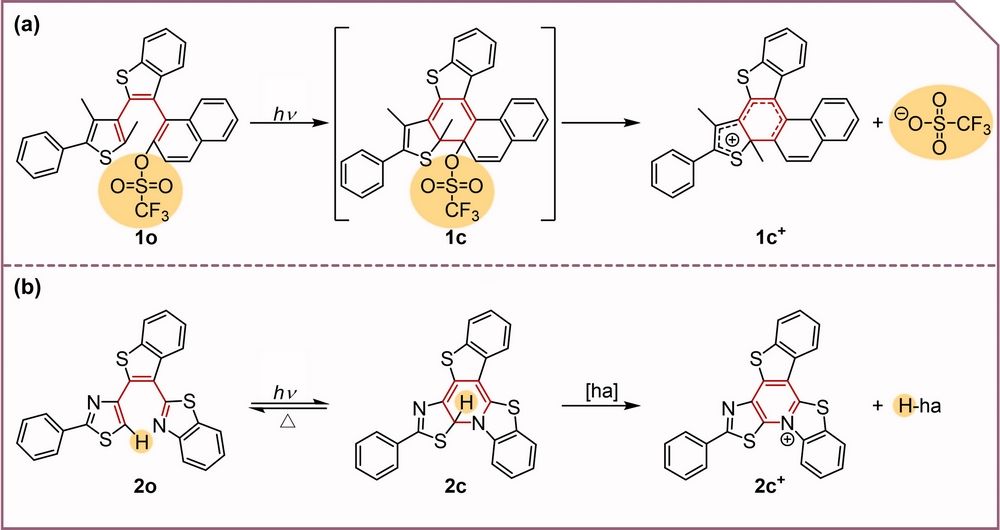
Figure 1. Photochemical reactions of (a) 1o and (b) 2o. “ha” refers to the “hydride acceptor”.
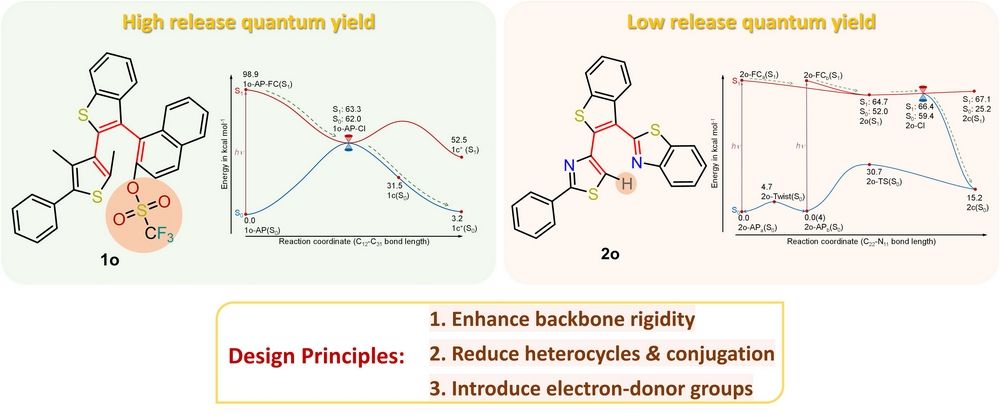
The different photochemical mechanisms are the key that leads to distinctive release quantum yields between 1o and 2o. For 1o, at room temperature, 1o-AP (reactive conformation) is the stable conformation, so 1o-AP is in majority. And the potential barrier of 1o-AP ® 1o-P (non-reactive conformation) is 3 kcal mol-1. In addition, according to the potential energy curve, the photochemical reaction of 1o-AP is barrierless (Fig. 2), which suppresses intersystem crossing (ISC). And the experimental results showed that the product 1c+ could be generated spontaneously. However, for 2o, 2o-AP is minority among all 2o conformers at room temperature and there is a potential barrier for the photochemical reaction (Fig. 3). In addition, experimental results indicated that 2c+ can only be observed in the presence of a hydride acceptor.
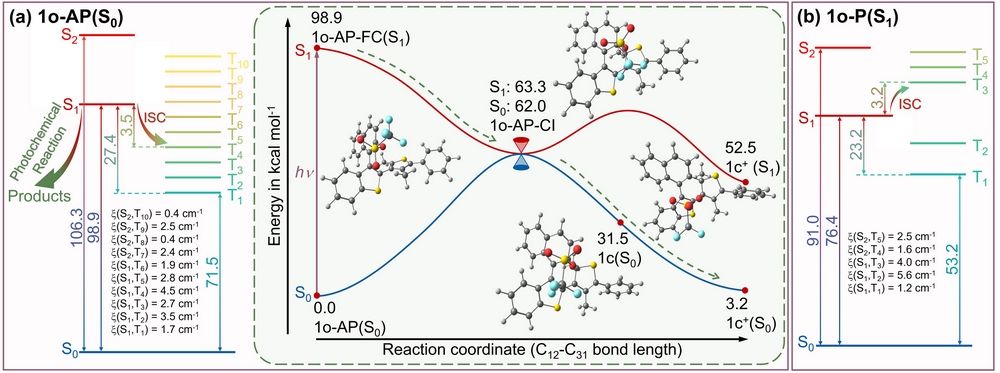
Figure 2. The calculated singlet and triplet states energy level diagram for (a) 1o-AP(S0) and (b) 1o-P(S1). Inset: PEC of 1o-AP. (the ground state energy of 1o-AP(S0) is used as zero for obtaining relative energies). [(TD)M062X/6-311G**/SMD (ACN)].
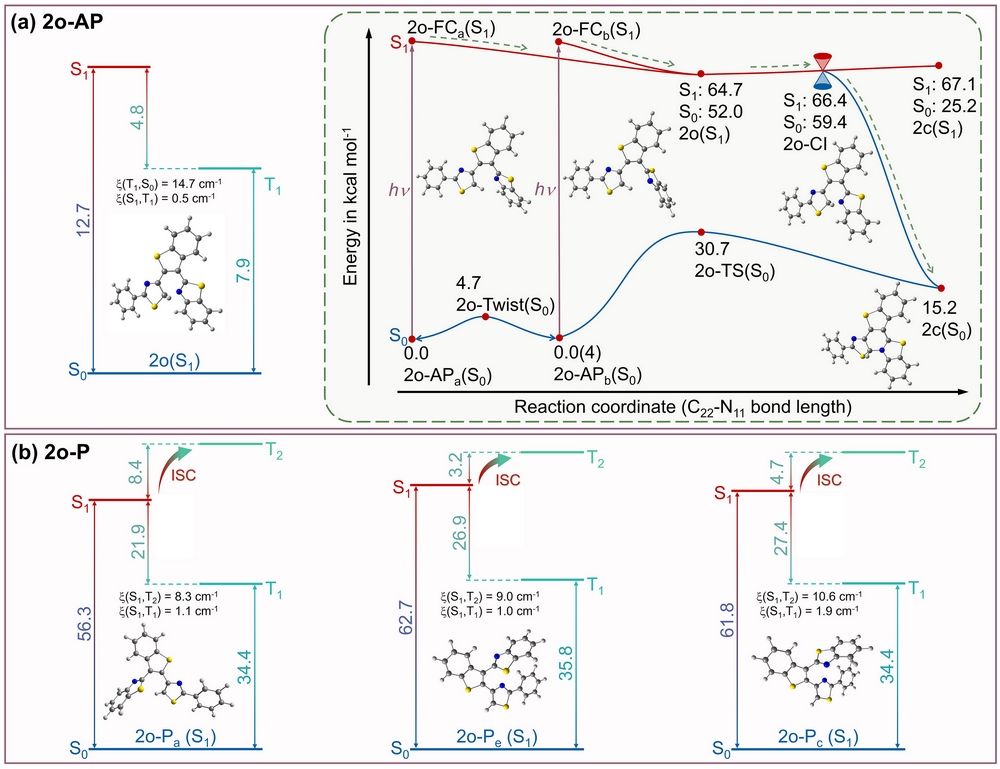
Figure 3. The calculated singlet and triplet states energy level diagram for (a) 2o-AP and (b) 2o-P. Inset: PEC of 2o-AP. (the ground state energy of 2o-APa(S0) is used as zero for obtaining relative energies). [(TD)M062X/6-311G**/SMD (THF)].
Based on the experimental and DFT/TD-DFT results, we propose the following ideas to improve the quantum yield of 2o. First, in order to stabilize 2o-AP(S0), enhancing the rigidity of the skeleton is a possible strategy. For example, the single bond rotation of the phenylthiazole and benzothiazole groups could be suppressed by introducing steric hindrance or an intramolecular hydrogen bond. Second, ISC competes with cyclization pathways in the singlet state, so decreasing the ISC efficiency is a feasible way to increase the quantum yield. The N and S-containing heterocycles usually give rise to enhanced spin orbit coupling strength and are beneficial for promoted ISC efficiency. The extended conjugating system lowers the energy of triplet states and offers more possible ISC channels for low-lying singlet states. In addition, it is expected that increasing the dehydrogenate capability of 2c by incorporating electron-donating groups would promote the 2c → 2c+ efficiency.
First Author: Su Yifan, doctoral candidate, Shaanxi Normal University
Correspondence Authors: Prof. Ma Jiani, Shaanxi Normal University; Assoc. Prof. Yu Le, Northwest University
Full Text Link: https://doi.org/10.1039/d4sc06202g
 Latest Updates
Latest Updates






